[ad_1]
This study is not affiliated with PCOS Challenge, Inc.
The announcement below is being provided for informational purposes only.

Receive up to $1,200 Monetary Compensation for Participation
See Below for Details and Eligibility Criteria
Study Title
The P.O.W.E.R. Study: Polycystic Ovary Syndrome Women Empowered Research
~1 in 3 women with PCOS make too much cortisol (the “stress” hormone) and male hormones
(DHEA, androstenedione and testosterone), and as a result, experience excess weight gain,
hair growth, acne, and irregular periods.
Eligibility Criteria
- Females between 18 to 40 years old
- Diagnosed with Polycystic Ovary Syndrome (PCOS)
- Not pregnant, breastfeeding, or trying to become pregnant in the next 6 months.
- Not diagnosed with either congenital adrenal hyperplasia (CAH) or Cushing’s syndrome
Trial Site Locations
22 clinical trial sites are available across the United States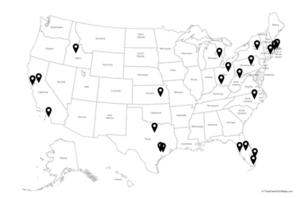
City, State
Los Angeles, CA
San Francisco, CA
New Haven, CT
Safety Harbor, FL
Saint Petersburg, FL
Saint Petersburg, FL
Miami, FL
West Palm Beach, FL
Winter Park, FL
Idaho Falls, ID
Wichita, KS
Fall River, MA
Quincy, MA
Baltimore, MD
Lancaster, NY
Cincinnati, OH
Cleveland, OH
Philadelphia, PA
Dallas, TX
Houston, TX
Morgan, WV
Estimated Time Commitment for Study Participants
- The study will take about 5 months to complete with 5 or 6 clinic visits and 3 phone calls over the first 4 months.
- There will be one clinic visit 4 weeks after the last dose of the investigational drug.
For Additional Information
*Disclaimer
[ad_2]
Source link



